Reflection:
Most valuable lesson:
The most valuable lesson that I learned this semester in chemistry was time management. Keeping up with all other classes and chemistry is definitely time consuming and requires a lot planning to ensure that study and homework time is used effectively.
Most challenging concept:
The most challenging chemistry concepts for me were the redox reactions; I had the most trouble setting up and balancing the equations.
Advice for future chem students:
I would advise future chemistry students to make sure that they take this course in a semester where they take on a light course load. This course requires a lot of time spent studying and doing the homework assignments, and the subject is not an easy one, so it would be much less stressful if students took this course alongside easier courses during the semester.
General Chemistry II: Campbellsville University
Caitlin Dresing's Chemistry Class Blog
Wednesday, May 1, 2013
Thursday, March 14, 2013
Fumarase
Fumarase or fumarate hydratase is an enzyme that catalyzes the
reversible reaction of hydration/dehydration of fumarate to malate. Fumarase
comes in two forms: mitochondrial and cytosolic. This enzyme participates in
three metabolic pathways; the Krebs Cycle (Cytric Acid Cycle), CO2
fixation and in renal cell carcinoma. This enzyme belongs to the family of
lysases, which cleave carbon-oxygen bonds.
Molecular Weight: 54,637 Da; 49,456.49 g/mol
Chemical Formula:
I was unable to locate a specifuc chemical formula for this enzyme from the web or from the Protein Data Bank website. However, I was able to find information as to how fumrase is typically found within a lab setting. Fumarase is typically extraccted from the porcine heart and is usually placed within a potassium phoshate suspension. This is often used in the lab to demponstrate the following raction process within the Krebs Cycle: L-Malate Fumarase > Fumarate + H2O
Structure:
Active Site Structure
3D
Rotatable Structure can be found on http://www.proteopedia.org/wiki/index.php/Fumarase
Reaction Catalyzed
The role of fumarase in the citric
acid cycle is to enable a transition step in the assembly of energy in the form
of NADH. In the cytosol, the enzyme metabolizes fumarate, which ends up as a
byproduct of the urea cycle as well as amino acid catabolism. Studies have
revealed that the active site is composed of amino acid residues from three of
the four subunits within the tetrameric enzyme. This process takes place in the
mitochondria.
Catalytic Activity:
(S)-malate = fumarate + H2O
Friday, February 8, 2013
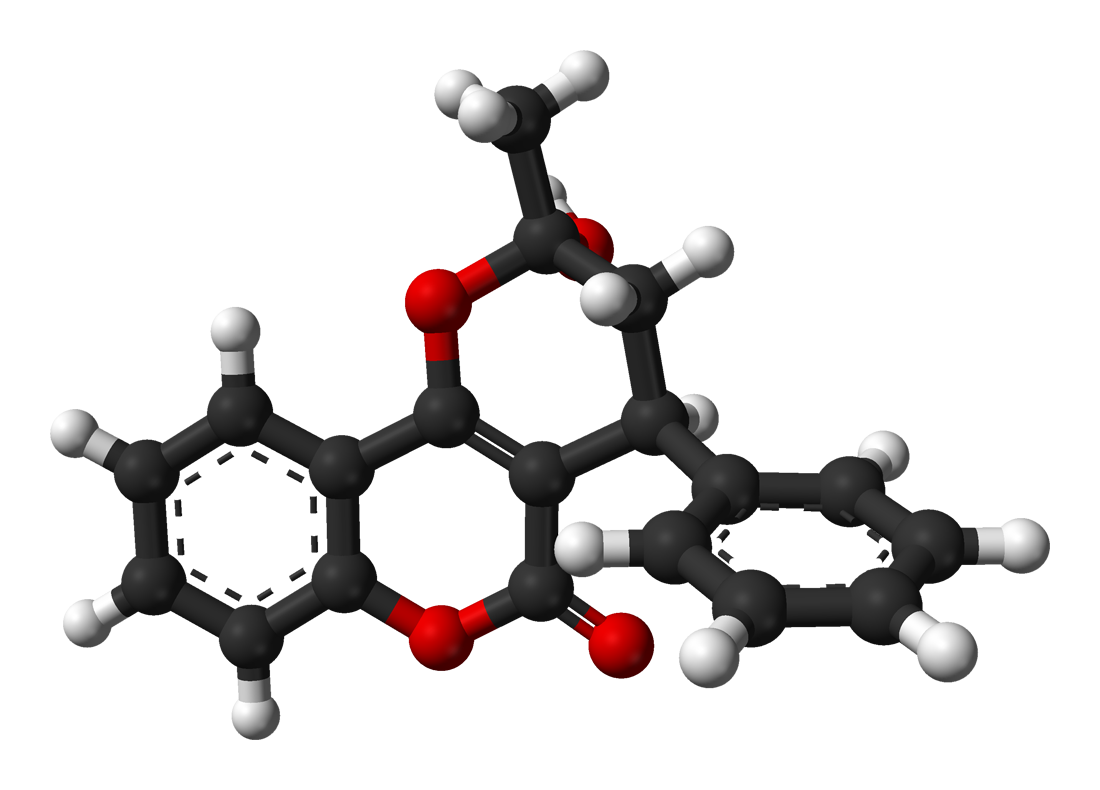
Warfarin
Warfarin is an anticoagulant also known as Coumadin. It is an odorless, colorless or white crystalline powder available commercially as a dust or liquid concentrate in various formulations. It is also found in anticoagulant medications and pesticides.

· IUPAC name: (RS)-4-hydroxy- 3-(3- oxo- 1-phenylbutyl)- 2H- chromen- 2-one
· 2-dimensional structure:
· 3-D Structure:
· Chemical formula: C19H16O4
· Molecular weight: 308.3 g/mol
· Physical properties
o Melting Point: 161⁰C
- Boiling points: Decomposes at 760 torr
- H2O solubility: Insoluble in H2O
- Benzene Solubility: Insoluble in Benzene
Intermolecular forces present in the molecule: Warfarin has dispersion forces, as well as dipole- dipole forces. It is capable of forming hydrogen bonds with other molecules. Warfarin also has 41 sigma bonds and 9 pi bonds.
Sources:
Subscribe to:
Comments (Atom)


.png)